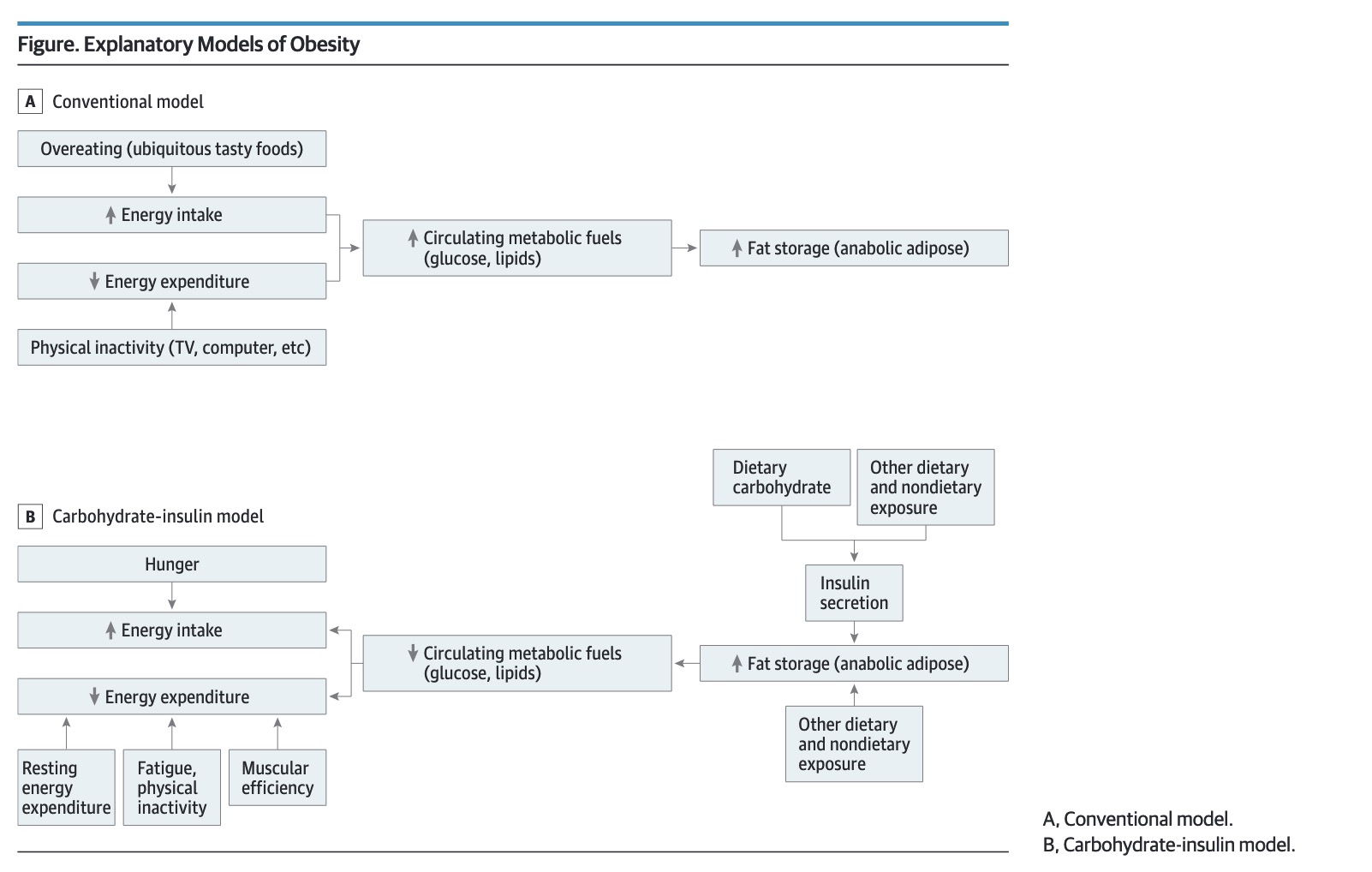
Despite intensive research, the causes of the obesity epidemic remain incompletely understood and conventional calorie-restricted diets continue to lack long-term efficacy. According to the carbohydrate-insulin model (CIM) of obesity, recent increases in the consumption of processed, high–glycemic-load carbohydrates produce hormonal changes that promote calorie deposition in adipose tissue, exacerbate hunger, and lower energy expenditure. Basic and genetic research provides mechanistic evidence in support of the CIM. In animals, dietary composition has been clearly demonstrated to affect metabolism and body composition, independently of calorie intake, consistent with CIM predictions. Meta-analyses of behavioral trials report greater weight loss with reduced-glycemic load vs low-fat diets, though these studies characteristically suffer from poor long-term compliance. Feeding studies have lacked the rigor and duration to test the CIM, but the longest such studies tend to show metabolic advantages for low-glycemic load vs low-fat diets. Beyond the type and amount of carbohydrate consumed, the CIM provides a conceptual framework for understanding how many dietary and nondietary exposures might alter hormones, metabolism, and adipocyte biology in ways that could predispose to obesity. Pending definitive studies, the principles of a low-glycemic load diet offer a practical alternative to the conventional focus on dietary fat and calorie restriction.
.